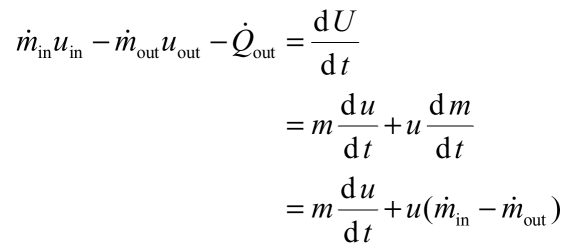Energy Balance Equation Thermodynamics Open System
In this video i derive the overall energy balance for an open and closed system involving shaft work work done by fluid flow and work done by expansion and compression.
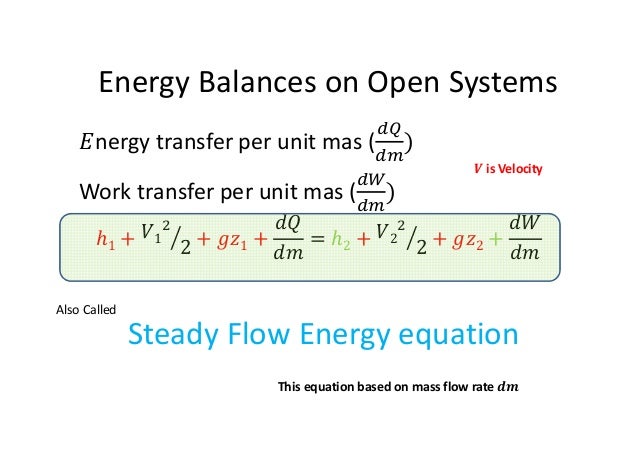
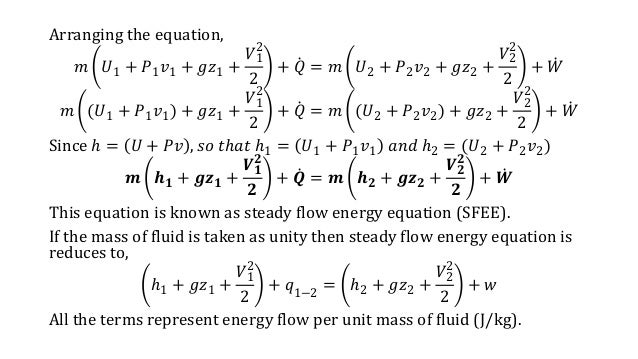
Energy balance equation thermodynamics open system. The energy involved with materials in transit is the enthalpy by definition. The big nasty energy balance equation at the bottom is the one we are most interested in right now. For energies in transit the energies transferring between system and surrounding only two types of energies are involved. By replacing u pv with h we obtain our energy balance or first law of thermodynamics for open systems.
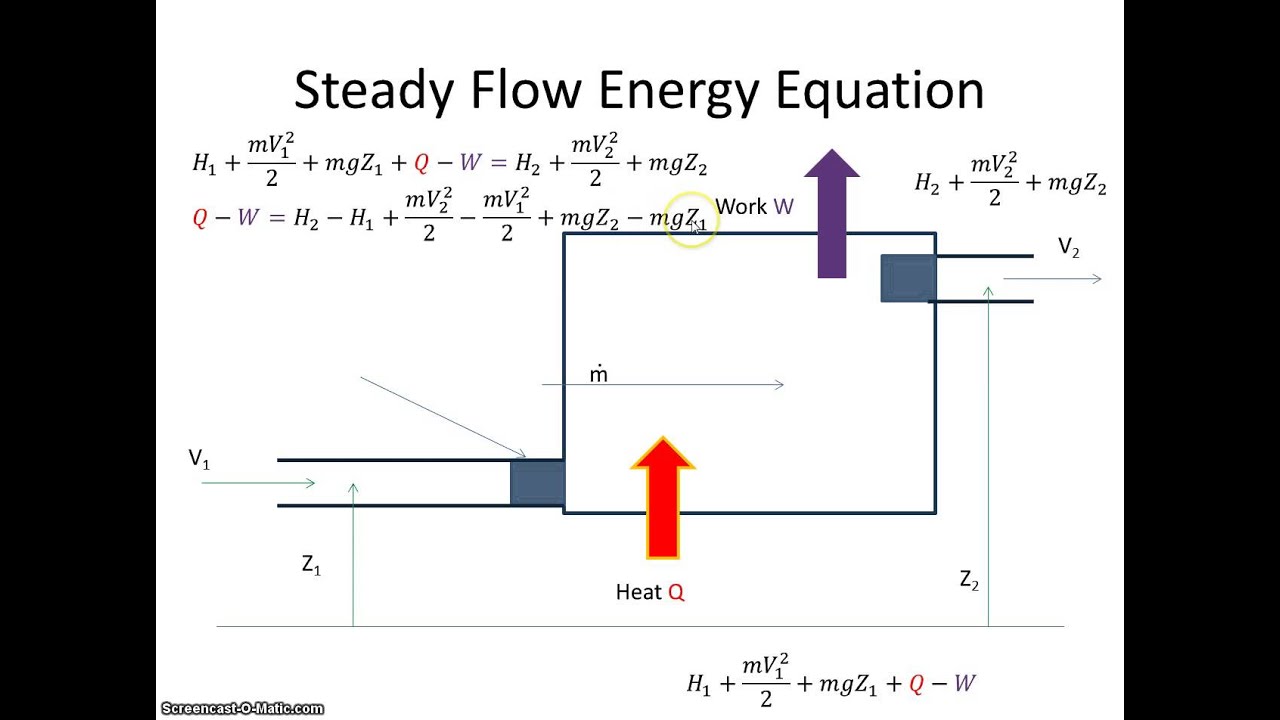
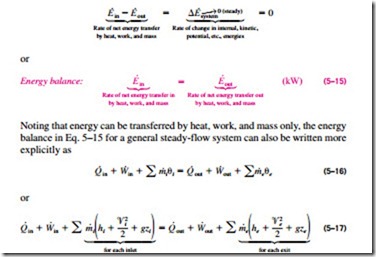
Then the energy balance for a uniform flow system can be expressed explicitly as where e h ke pe is the energy of a flowing fluid at any inlet or exit per unit mass and e u ke pe is the energy of the nonflowing fluid within the control volume per unit mass. For example living systems are clearly able to achieve a local reduction in their entropy as they grow and develop. This equation has a. The general energy balance for a process can be expressed in words as.
This video is from the free online course. For that reason their sum has been given the name enthalpy en. The heat and work. Enthalpy is also a thermodynamic property.
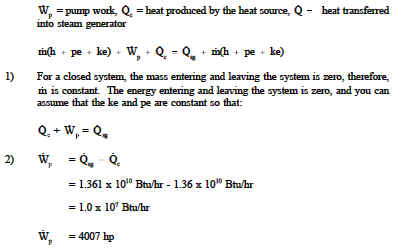
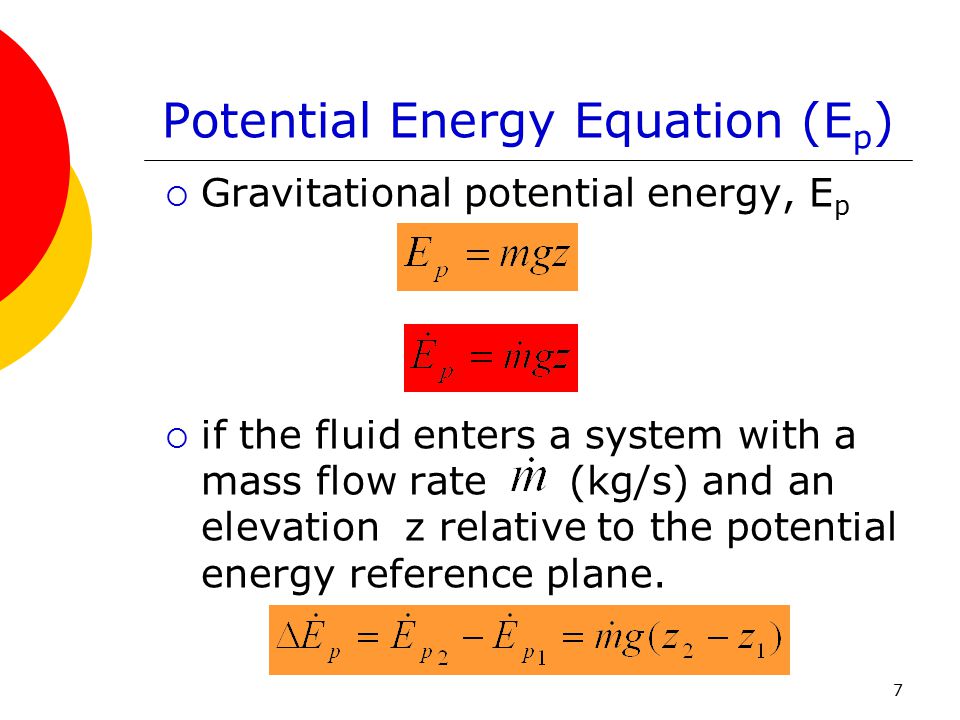
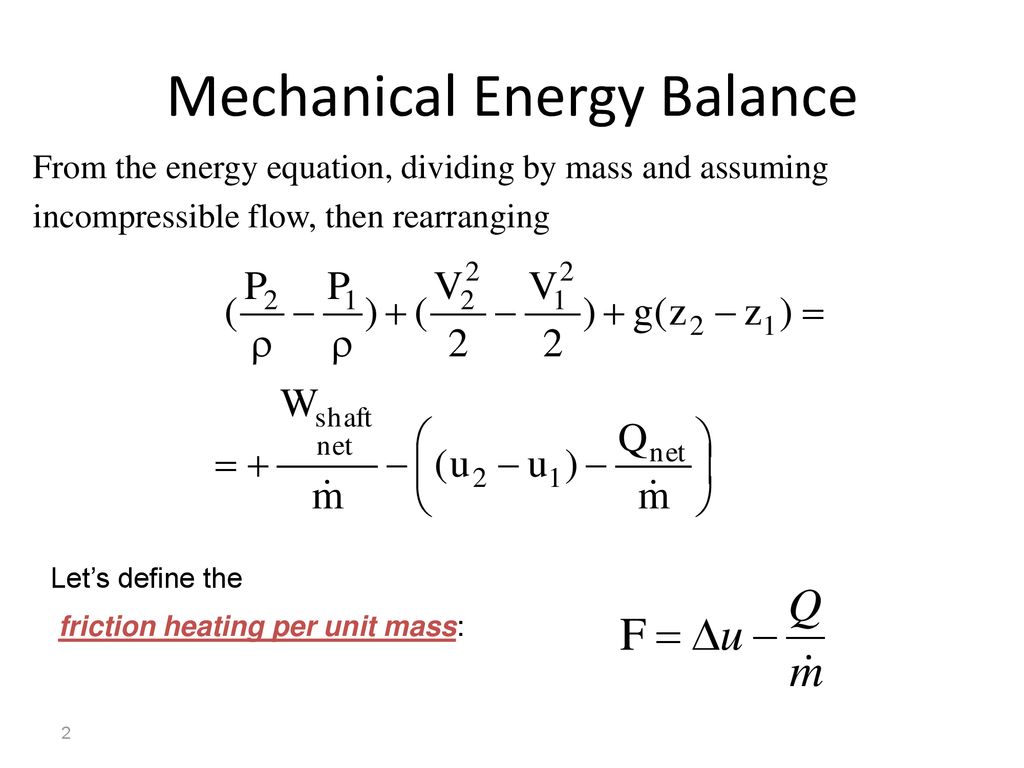
Accumulation of energy in system input of energy into system output of energy from system 6 now the total energy of a system as considered above is composed of kinetic potential and internal energies. It s the equation that is most similar to the entropy balance equation we are going to write. In energy balance equation for the closed system the energy change of the system is described as the energy change of internal energy potential energy and the kinetic energy. The application of thermodynamics to pump systems 2 5 the energy balance is.
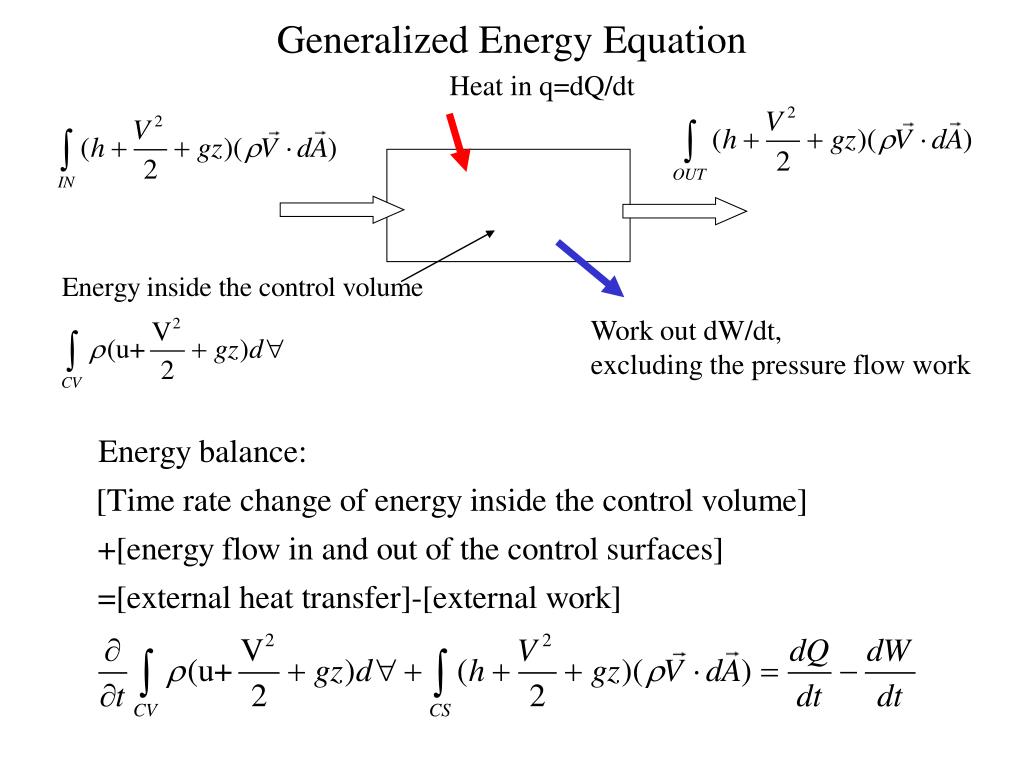
Here is a quick review of mass and energy balances for open and closed systems. Thermodynamics thermodynamics open systems. Most real thermodynamic systems are open systems that exchange heat and work with their environment rather than the closed systems described thus far. 2 4 open systems enthalpy kinetic and potential energy.
Ch 8 lesson b page 3 mass energy balances. The question was concerning the energy equation for an open system. They create structures of greater internal energy i e they lower entropy. D e d t rate of change of total energy in the system δ q rate of heat transfer δ w.
The energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy transfer accompanied with materials transferring across the system boundary.