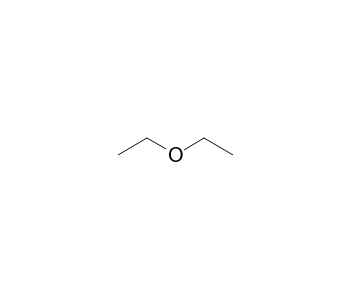
Diethyl Ether Has The Following Structure
It is commonly used as a solvent in laboratories and as a starting fluid for some engines.
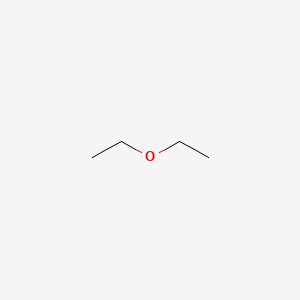
Diethyl ether has the following structure. Get more help from chegg. So in pure dimethyl ether hydrogen bond does not occur. Diethyl ether has the following structure. Based on the information so far the structural formula is.
Get 1 1 help now from expert chemistry tutors. It is a colorless highly volatile sweet smelling ethereal odour flammable liquid. Diethyl ether e none of the above. Give the structure that corresponds to the following molecular formula and 1h nmr spectrum.
Here no hydrogen atom is attached to oxygen atom. Ether c2h5 2o or c4h10o cid 3283 structure chemical names physical and chemical properties classification patents literature biological activities. Ethyl ether also known as diethyl ether well known anesthetic generally referred to as simply ether an organic compound belonging to a large group of compounds called ether. Diethyl ether or simply ether is an organic compound in the ether class with the formula c 2 h 5 2 o sometimes abbreviated as et 2 o see pseudoelement symbols.
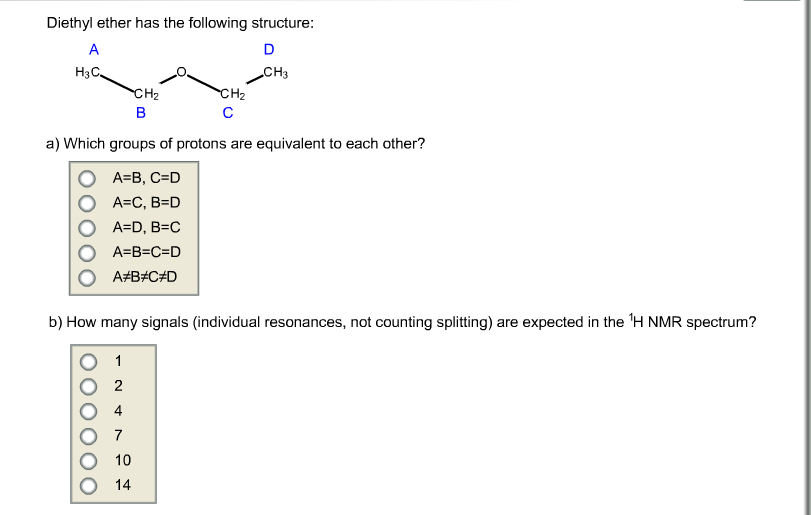
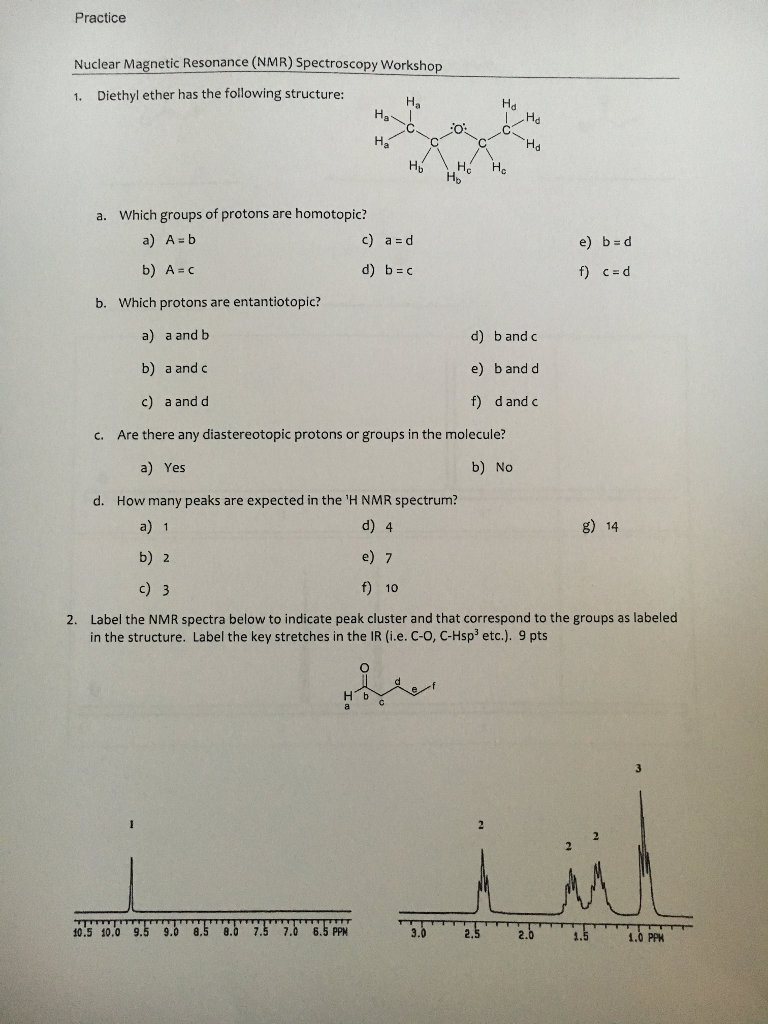
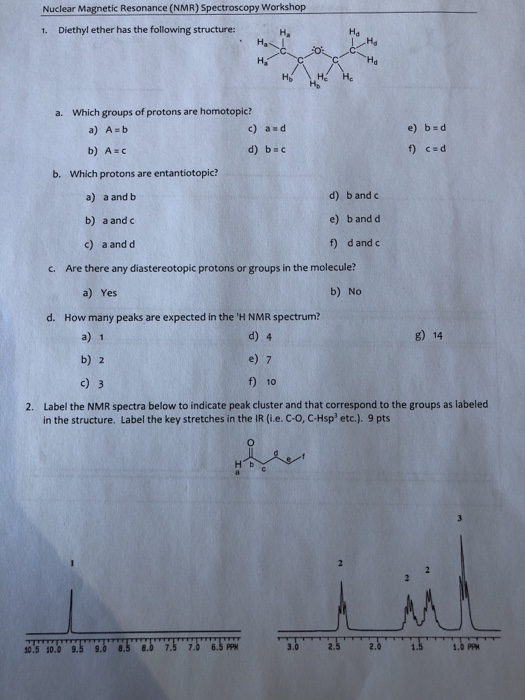
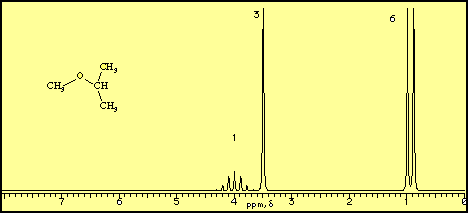
C methyl ethyl ether which of the following compounds is most soluble in water a ch3 ch2. Label each proton with the predicted splitting pattern it would exhibit in a 1h nmr spectrum. Its molecular structure consists of two ethyl groups connected by an oxygen atom as in c2h5oc2h5. Diethyl ether has the following structure which groups of protons are equivalent to each other.
Ether suggests that you are looking at a compound with the general formula r 1 o r 2. The structure of the dimethyl ether is as follows. What class of compound has the following general formula o r c o r a ester b carboxylic acid c ketone d aldehyde e phenol. Ethyl suggests that at least one of the functional groups which is bonded to the oxygen is well ethyl c 2 h 5 di ethyl reveals that not just one but two of the functional groups are in fact ethyl radicals.
How many signals individual resonances not counting splitting are expected in the 1h nmr spectrum.